Archives
- 2018-07
- 2018-10
- 2018-11
- 2019-04
- 2019-05
- 2019-06
- 2019-07
- 2019-08
- 2019-09
- 2019-10
- 2019-11
- 2019-12
- 2020-01
- 2020-02
- 2020-03
- 2020-04
- 2020-05
- 2020-06
- 2020-07
- 2020-08
- 2020-09
- 2020-10
- 2020-11
- 2020-12
- 2021-01
- 2021-02
- 2021-03
- 2021-04
- 2021-05
- 2021-06
- 2021-07
- 2021-08
- 2021-09
- 2021-10
- 2021-11
- 2021-12
- 2022-01
- 2022-02
- 2022-03
- 2022-04
- 2022-05
- 2022-06
- 2022-07
- 2022-08
- 2022-09
- 2022-10
- 2022-11
- 2022-12
- 2023-01
- 2023-02
- 2023-03
- 2023-04
- 2023-05
- 2023-06
- 2023-08
- 2023-09
- 2023-10
- 2023-11
- 2023-12
- 2024-01
- 2024-02
- 2024-03
- 2024-04
-
Circular dichroism CD provides useful information about prot
2024-04-25
Circular dichroism (CD) provides useful information about protein structure, the extent and rate of structural changes and ligand binding. CD is a form of light ZLN005 spectroscopy that measures differences in the absorbance of right- and left-circularly polarized light (rather than the commonly us
-
Furthermore to address the concern that the observed
2024-04-25
Furthermore, to address the concern that the observed positive in vitro activity results might be stymied by the propensity of such assays to display false positives due to the inherent noxiousness of the tested compounds, all analogs were evaluated against Vero cells in an MTT assay. Some of the mo
-
sphk A unique finding in this study is
2024-04-25
A unique finding in this study is that 12/15-LO influences CREB phosphorylation in the murine brain. While previous reports have found that knockout of 5-lipoxygenase, a related enzyme that also uses arachidonic sphk for substrate, can regulate CREB phosphoryation in the context of Alzheimer's dise
-
br Materials and methods br Results br Discussion In this
2024-04-25
Materials and methods Results Discussion In this study, we have demonstrated that the adiponectin system (genes and proteins) is present in the porcine endometrium (epithelial glandular cells, luminal epithelial Topiroxostat and stromal cells) and myometrium (longitudinal and circular musc
-
br Hyperadiponectinemia in disease conditions Other evidence
2024-04-25

Hyperadiponectinemia in disease conditions Other evidence indicates that hyperadiponectinemia does not necessarily always imply a healthy outcome. Indeed, a recent study suggested that hyperadiponectinemia occurs in various diseases. Given that the risk for AD and vascular dementia is increased i
-
Our co IP data show that HT A or
2024-04-25
Our co-IP data show that 5HT1A or 5HT2A receptors do not heterodimerize with mGluR1α receptors. Given that both 5-HT and mGlu receptors are GPCRs and produce anxiolytic effects, a degree of cooperativity via a functional cross-talk may still exist between these receptors [11]. In addition to the fun
-
Adenosine receptors activate a number of signalling
2024-04-25
Adenosine receptors activate a number of signalling pathways involved in tissue survival including several mitogen-activated protein kinases (Fredholm et al., 2001). The common feature of all adenosine receptors is the positive coupling to ERK1/2, whilst A2B and A3 receptors can also activate the st
-
Toxicity is the main reason for the failure at
2024-04-25

Toxicity is the main reason for the failure at all stages of the new drug development process. The major part of safety-related attrition occurs at preclinical phases while predicting preclinical safety liabilities earlier in the drug development process. This strategy enables the design and/or sele
-
br Acknowledgments The authors would like to
2024-04-25
Acknowledgments The authors would like to thank Ms. Ashley Davis for her administrative assistance in preparing this article and Mr. Dan Beck for his artistic contributions to Fig. 1. Introduction Acetylcholine is the most abundant neurotransmitter in the central nervous system (CNS) of insec
-
Although effects of low concentrations
2024-04-25
Although effects of low concentrations of agonist were not as thoroughly documented for heteromeric receptors such as the major Triflusal synthesis α4β2 nAChR, a similar mechanism of action was described to explain the potentiation of these receptors with low concentrations of acetylcholine-esteras
-
As also found by Stolarski et al inclusion of
2024-04-25
As also found by Stolarski et al. [14], inclusion of N2O in the model is successful in reducing the effect of dynamics [13] in the lower stratosphere in the Northern Hemisphere (30–60°N in our case). The inclusion of N2O also decreased the error bars on the trend values, but the trend values still c
-
Caffeic acid dihydroxycinnamic acid is a natural compound th
2024-04-25
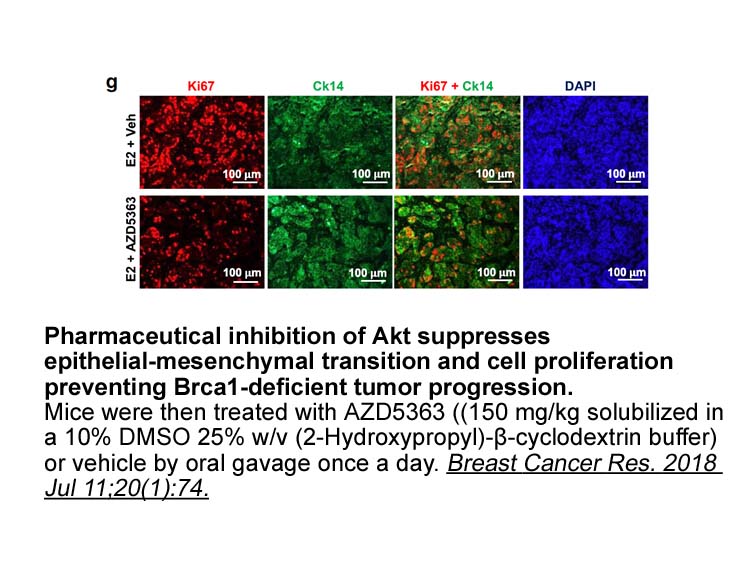
Caffeic Cy3 NHS ester (non-sulfonated) (3,4-dihydroxycinnamic acid) is a natural compound that inhibits 5-LO and exerts potent anti-inflammatory and antioxidant properties. Recently, Takeda et al. reported that caffeic acid provided neuroprotective and anti-depressive activities [23]. Sertraline, a
-
The discovery of acetylsalicylic acid aspirin in paved the w
2024-04-25
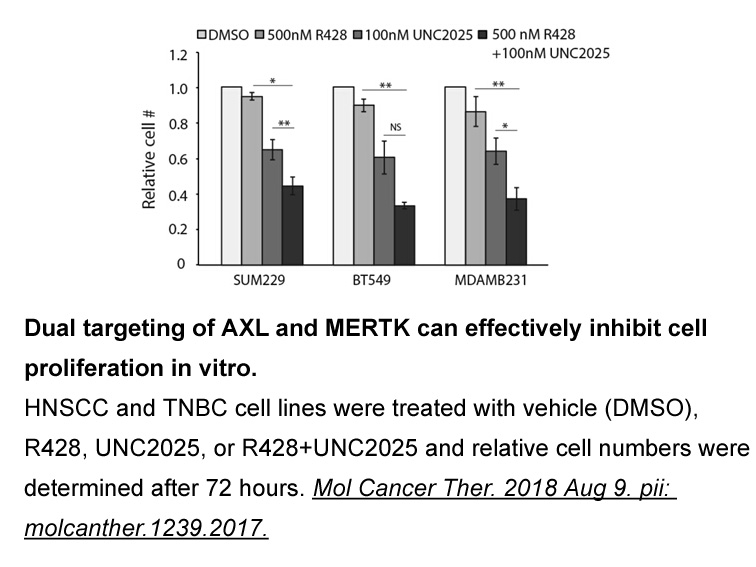
The discovery of acetylsalicylic gsk525762 synthesis (aspirin) in 1897 paved the way for the development of classical NSAIDs as first-line therapeutics for anti-inflammatory, anti-pyretic and analgesic therapy. Large efforts have been made in the following decades to improve the efficacy and in par
-
The observation that vortioxetine blocks HT induced currents
2024-04-25
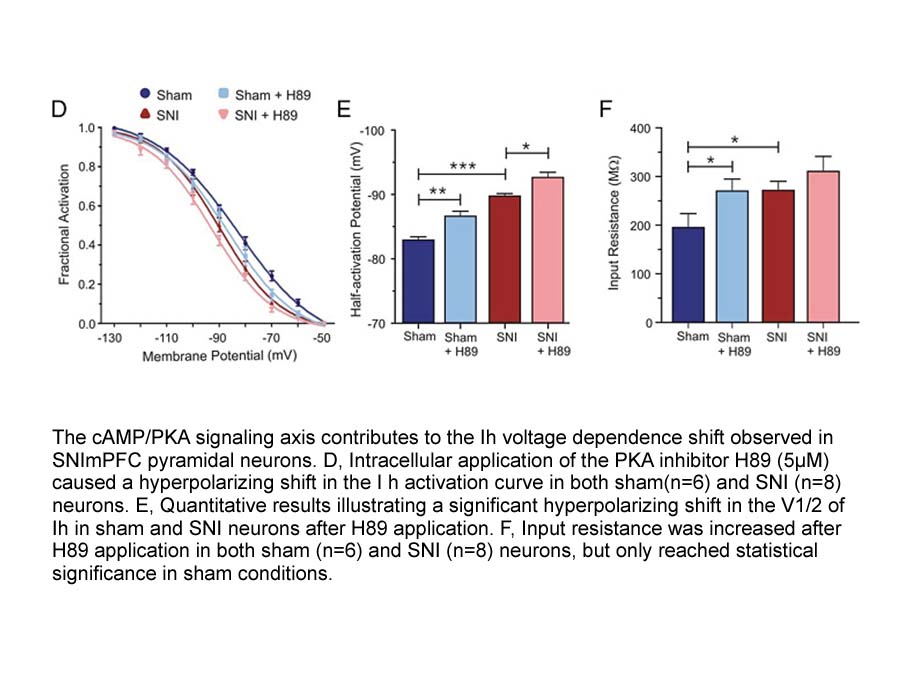
The observation that vortioxetine blocks 5-HT-induced currents in 5-HT3 receptor-expressing oocytes suggests that vortioxetine acts to functionally antagonize these receptors, and is in line with previously published data (Bang-Andersen et al., 2011). For example, Mørk et al. (2012) demonstrated tha
-
br ARIs in the prevention of prostate
2024-04-25
5ARIs in the prevention of prostate cancer Prostate cancer continues to be a leading cause of male deaths worldwide. In 2009, it was estimated there would be 192,280 new cases of prostate cancer with a predicted 27,360 deaths [25]. Because androgens, and specifically DHT, play a large role in bot